
CureGene Publishes Research Findings in European Journal of Medicinal Chemistry
Recently, CureGene published a research article titled "Discovery of A Novel and Potent Antiplatelet Thiol Prodrug CG-0255" in European Journal of Medicinal Chemistry, a leading journal in the field of medicinal chemistry. The paper systematically presents the complete drug discovery and optimization process of CG-0255, a world’s first esterase mediated thiol hydrolysis prodrug, and provides the first comprehensive disclosure of its pharmacokinetic and antiplatelet efficacy data based on thiol prodrug technology.
Cardiovascular and cerebrovascular diseases are the leading cause of death world widely, posing a serious threat to human health. Artery thrombosis and subsequent blockage of the arteries are the direct causes for heart attacks and strokes, platelet activation and aggregation are the most critical factors in thrombin formation. Therefore, P2Y12 antagonists play an essential role in the prevention and treatment of coronary heart disease.
Clopidogrel has been the most widely prescribed antiplatelet drug for more than 20 years, however, it has significant drawbacks. It is a prodrug and requires multiple oxidative metabolic steps via the hepatic CYP450 enzymes, CYP2C19 in particular, for conversion into its active metabolite (H4). This leads to several major clinical issues. Firstly, there is significant portion of world patient population with loss of function CYP2C19 alleles, which results in low or no drug effect, this well recognized phenomenon is called clopidogrel resistance and is prominently displayed in US FDA label with a black boxed warning. Secondly, the drug’s potency is relatively weak, averaging ~50%, leading to lower efficacy. Thirdly, it has a slow onset of action, making it unsuitable for rapid intervention in acute thrombotic events, greatly limits its effectiveness in complex clinical settings.
CureGene's research demonstrates that CG-0255 exhibits potent platelet inhibition and excellent metabolic stability in vitro. Further, CG-0255 relies on a faster hydrolytic process for conversion to H4, therefore completely bypassing CYP metabolic pathway and overcoming clopidogrel resistance, laying a solid foundation for its clinical success. Pharmacokinetic analyses reveal a strong positive correlation between exposure to the active metabolite and administered doses. Pharmacodynamic evaluations confirm a clear dose-dependent inhibition of platelet aggregation with fast onset of action and much higher potency. Thanks to innovative structural optimization, CG-0255 has been developed into dual formulations, oral and IV injection. IV formulation fills worldwide market vacancy in stroke while oral formulation can address all clinical settings, making it possible to fulfil diverse unmet clinical needs and demonstrating broad potential for clinical uses.
Original Article: https://www.sciencedirect.com/science/article/abs/pii/S022352342500738X
CG-0255 is a first-in-class thiol-based prodrug derived from H4. It undergoes a single hydrolysis step to generate H4, bypassing the need for CYP450 enzyme-mediated metabolism, thereby overcoming the issue of "clopidogrel resistance." Additionally, due to CG-0255’s favorable stability and solubility, it can be developed into an injectable formulation, meeting clinical needs in emergency settings or for patients unable to take oral medication. In vitro and in vivo studies indicate that CG-0255 is primarily hydrolyzed by carboxylesterase (CES) to produce H4. Both oral and intravenous administration result in rapid antiplatelet effects in vivo, with responses showing clear dose dependency.
Research Methods
Liver microsomal stability assays showed that the addition of coenzyme NADPH did not affect the intrinsic clearance rate (CLint) of CG-0255, confirming its independence from CYP450 metabolism. Further investigation identified carboxylesterase (CES) as the key enzyme responsible for the hydrolysis of CG-0255.
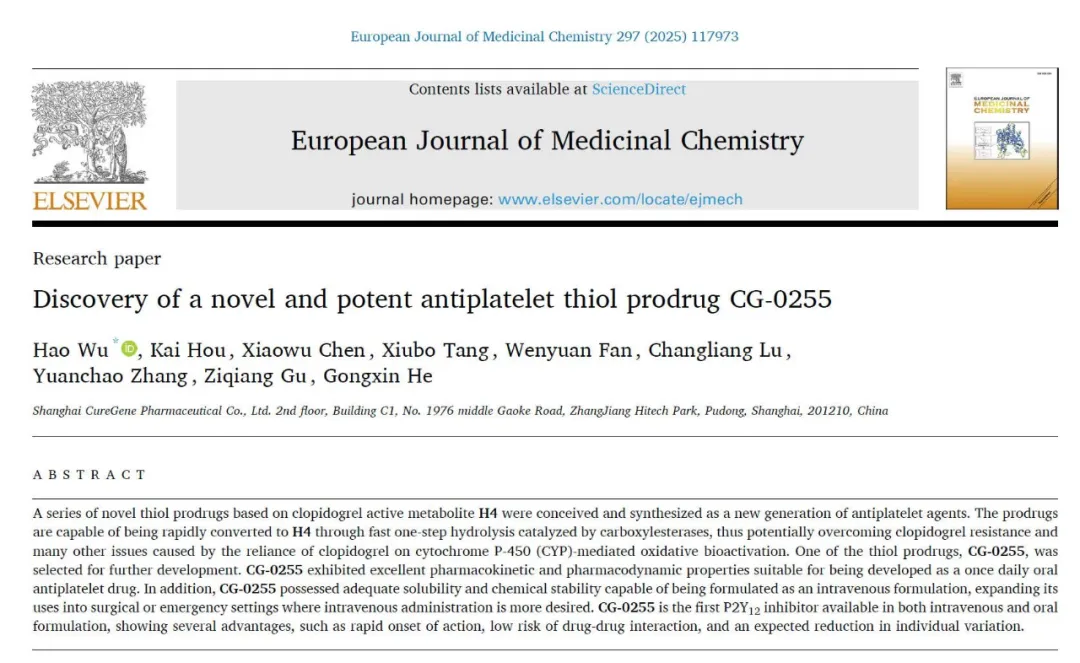
Pharmacokinetic and pharmacodynamic studies in beagle dogs demonstrated that after either oral or intravenous administration of CG-0255, the active metabolite H4 was rapidly generated in vivo (Figure 1), resulting in immediate antiplatelet activity (Figure 2). Both systemic exposure to H4 and platelet inhibition rates (IPA%) were dose-dependent. After oral administration, CG-0255 achieved equivalent H4 exposure at just one-tenth the dose of clopidogrel. Both routes of administration showed onset of action within 10 minutes and reached maximum antiplatelet effect within 30 minutes.
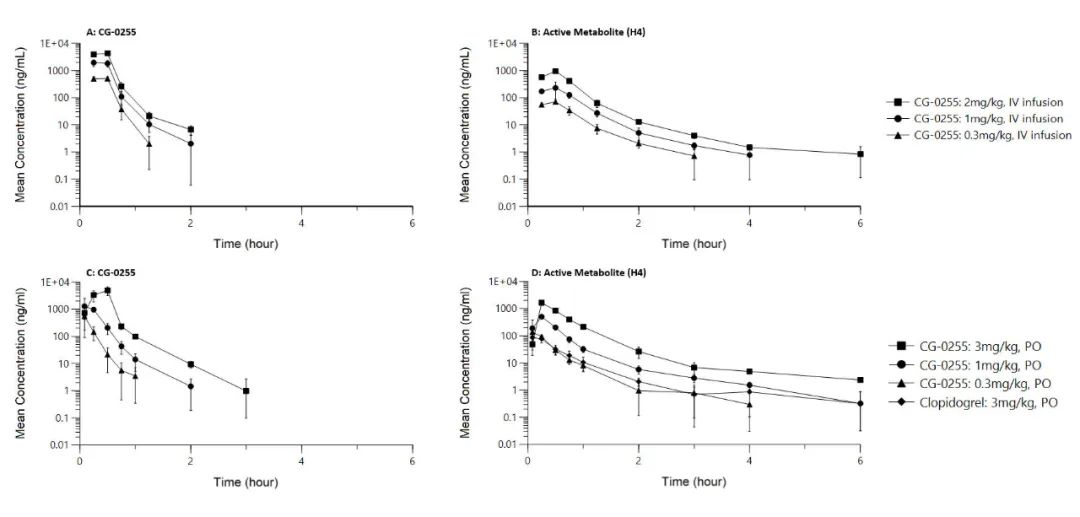
Figure 1: Concentration-Time Curve in Beagle Dog Studies
Figure 2: Inhibition Rate of Platelet Aggregation of CG-0255 in Beagle Dog
CG-0255 is a novel thiol prodrug designed based on the active moiety H4. Its core innovation lies in circumventing the complex oxidative metabolic pathway required by clopidogrel. Upon entering the body, CG-0255 is rapidly hydrolyzed in a single step by carboxylesterase (CES)—an enzyme widely present in humans—to release the active compound H4.
The study comprehensively demonstrates the advantages of CG-0255 through both in vitro and in vivo experiments:
1.Rapid Onset and Low Inter-Patient Variability
In beagle dog studies, both oral and intravenous administration of CG-0255 resulted in therapeutic effects within 10 minutes, reaching peak antiplatelet activity within 30 minutes. Due to its simple and direct metabolic pathway, CG-0255 avoids the problem of "clopidogrel resistance," reducing inter-patient variability and the risk of drug-drug interactions.
Preclinical data show that oral CG-0255 achieves the same level of active H4 exposure—and comparable antiplatelet effects—as clopidogrel, but at only one-tenth the dose. This offers superior efficacy potential, reduced risk of side effects, and improved pharmacoeconomic value.
Currently, Phase III registration clinical trials of CG-0255 are underway in the United States, and Phase II trials are ongoing in China. CG-0255 holds tremendous promise as a new and superior treatment option, particularly for patients facing clopidogrel resistance or in acute stage of heart attacks and strokes, requiring fast onset of actions and convenience of IV dosing.
CG-0255 is a next-generation, revolutionary antiplatelet agent featuring both intravenous and oral formulations. It is currently in clinical development for indications including acute coronary syndrome, recent myocardial infarction, recent stroke, and peripheral artery disease - aimed at addressing unmet clinical needs and providing better antiplatelet solutions for a broad patient population. In clinical practice, CG-0255 enables rapid onset of action, demonstrates a favorable safety profile, exhibits minimal inter-individual variability, and is largely unaffected by drug-drug interactions. It has the potential to become the leading P2Y12 receptor antagonist and fully meet clinical demands across diverse treatment scenarios.
CureGene was founded in 2018. Based in China, we strive to become a globally focused innovative biopharmaceutical company. We have established innovative platforms based on our core expertise and capability. Currently we are focused on cardio-cerebrovascular and antiviral disease areas. We have developed a number of pipelines of drugs with huge market potential and fully owned global IP rights and are actively moving these projects forward. Guided by our shared mission statement, we have assembled a group of talented and highly accomplished scientists with global pharmaceutical experience from China and overseas. With a comprehensive strategic vision, CureGene has successfully and highly efficiently moved from research stage startup into a clinical stage company, with all pipelines capable of First-in-Class or Best-in-Class potential.